The Alliance for the Prudent Use of Antibiotics (APUA) is proud to announce the launch of the third batch of ‘Antibiotic cards’ for a further five antibiotics currently in use. This project aims to provide a summary of the main characteristics of selected antibiotics. These antibiotic cards have been primarily designed to assist the prescribers in the selection of the most appropriate antibiotic, with optimal dosages, and to provide guidance to those who take care of patients who receive antibiotics.
These antibiotic cards have been produced by members of the APUA board and colleagues, with no support from the pharmaceutical industry, and are available free of charge. They share a common design, with a special focus on their antimicrobial spectrum, main indications, dosing, and adverse events. We hope that you will find them useful and that these antibiotic cards will contribute to better use of antibiotics worldwide, the primary goal of APUA.
Click the individual images below to view and download the cards separately or visit the download page.
Batch 3
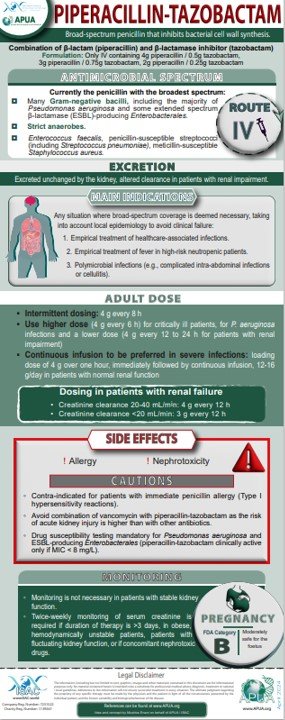
Cefepime, Ceftazidime-Avibactam, Ciprofloxacin, Levofloxacin, Piperacillin-Tazobactam
Batch 2
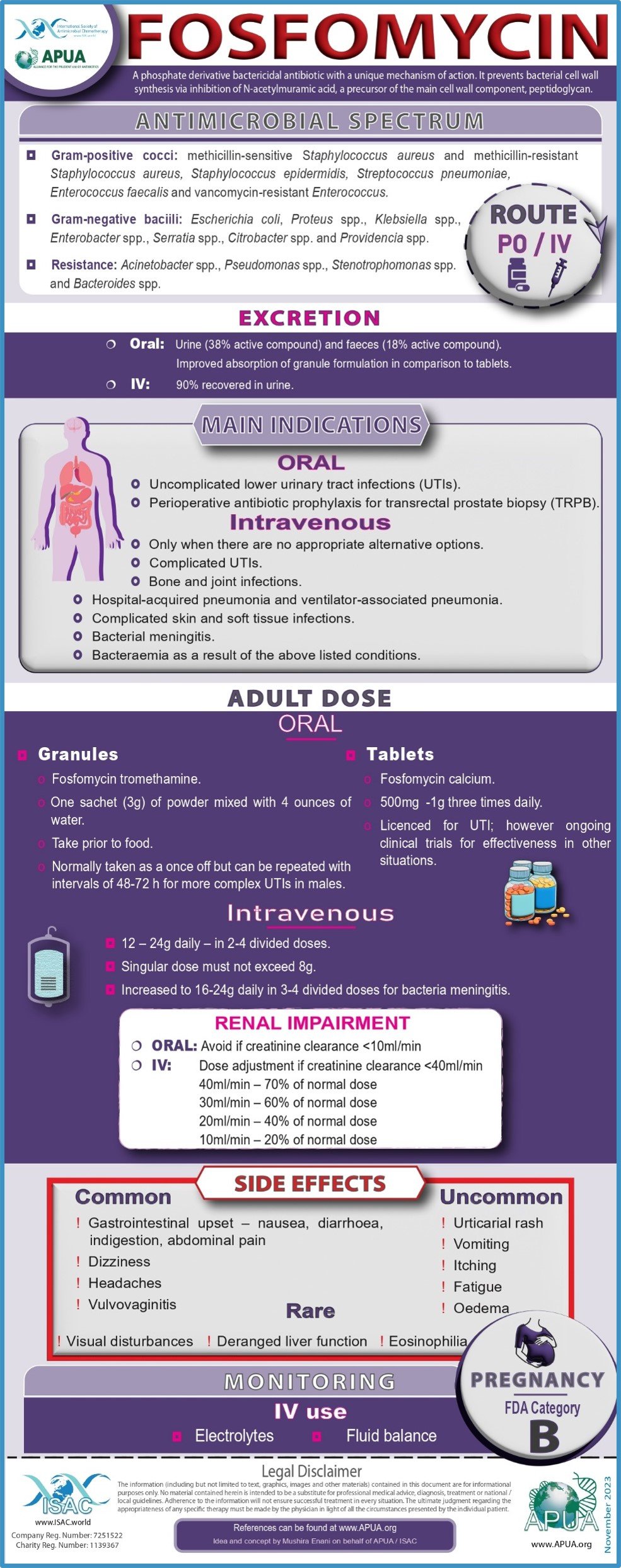
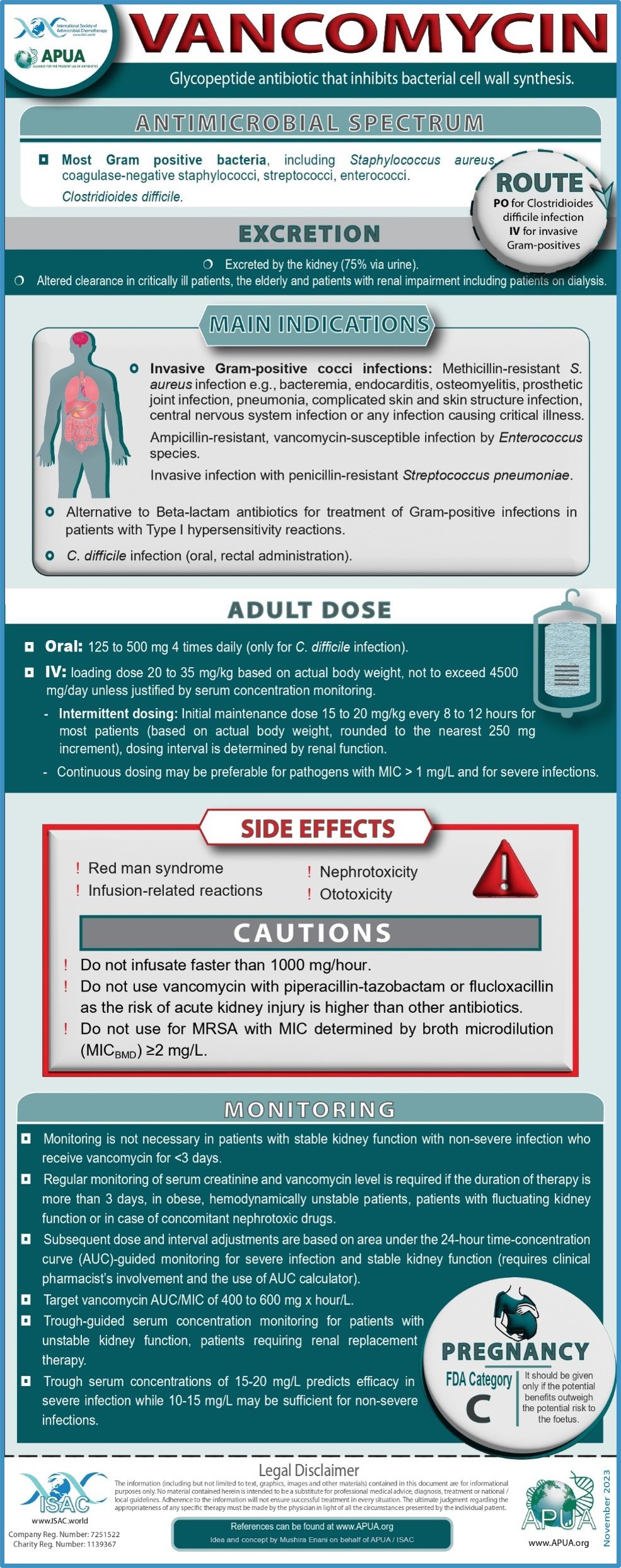
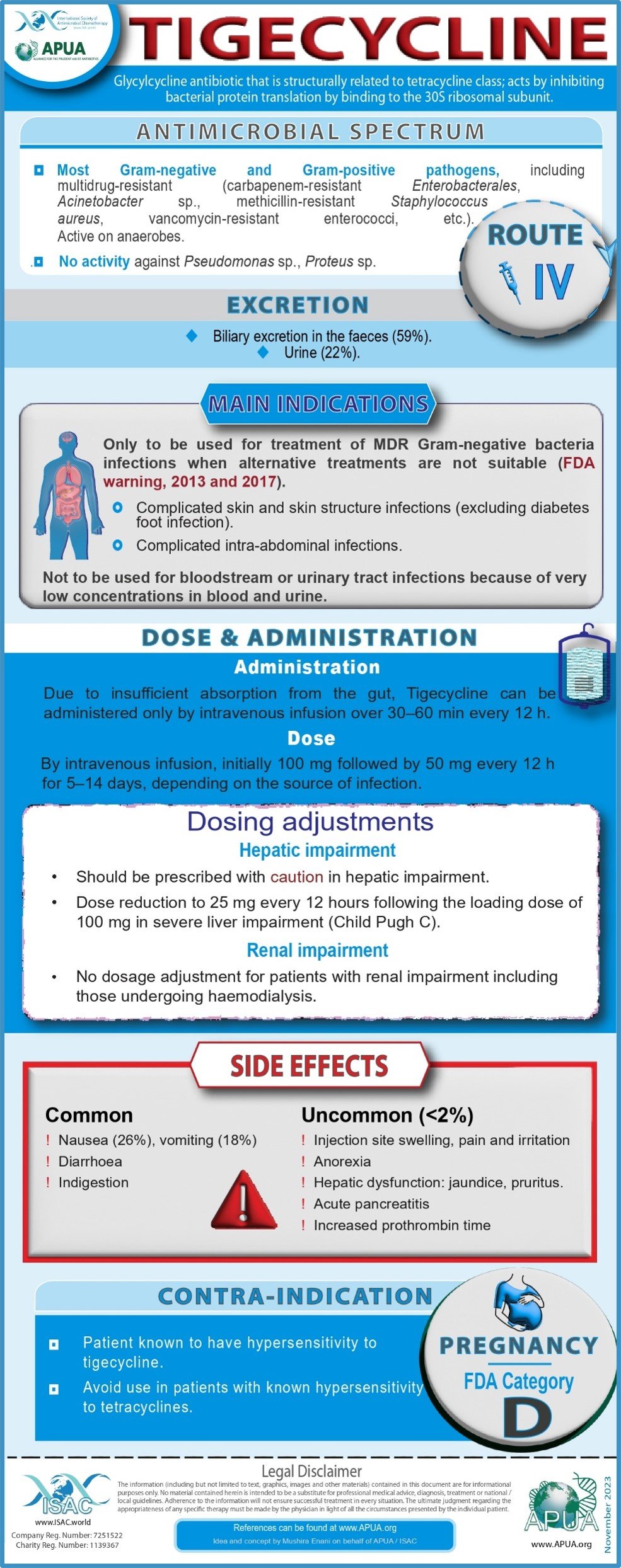
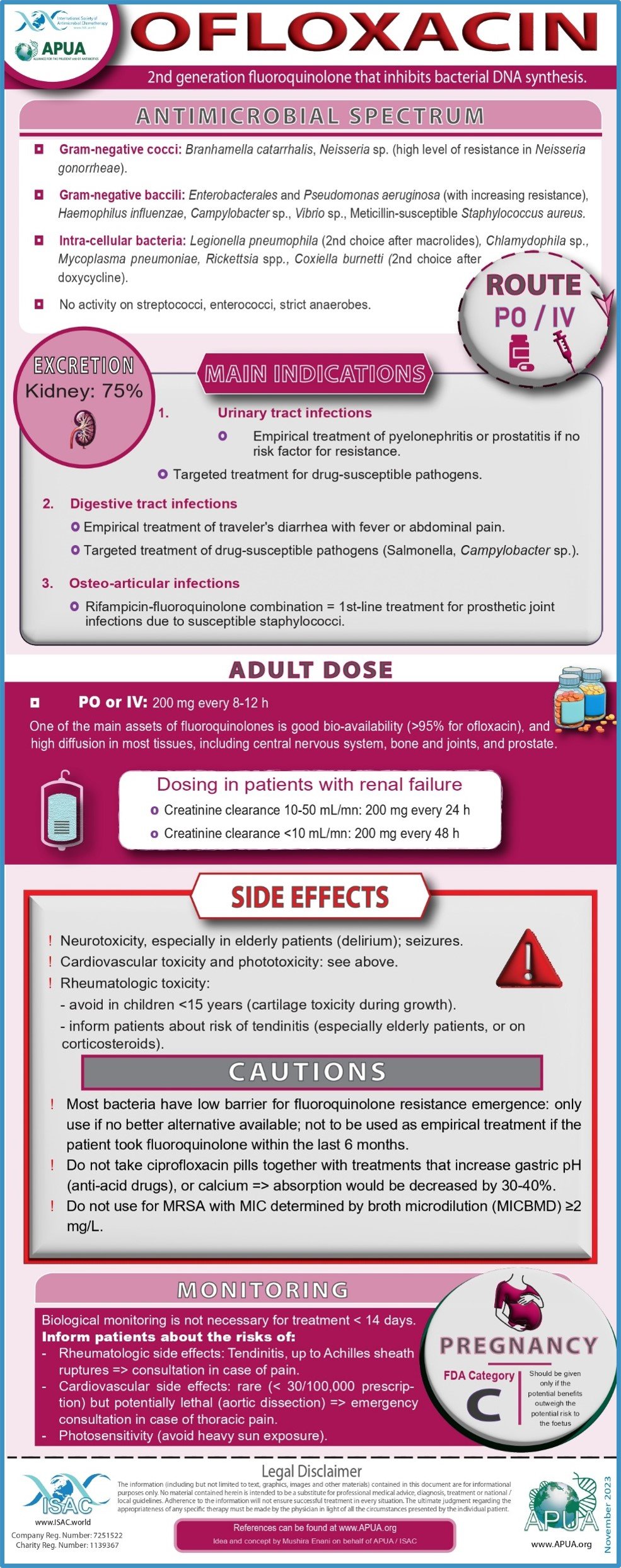
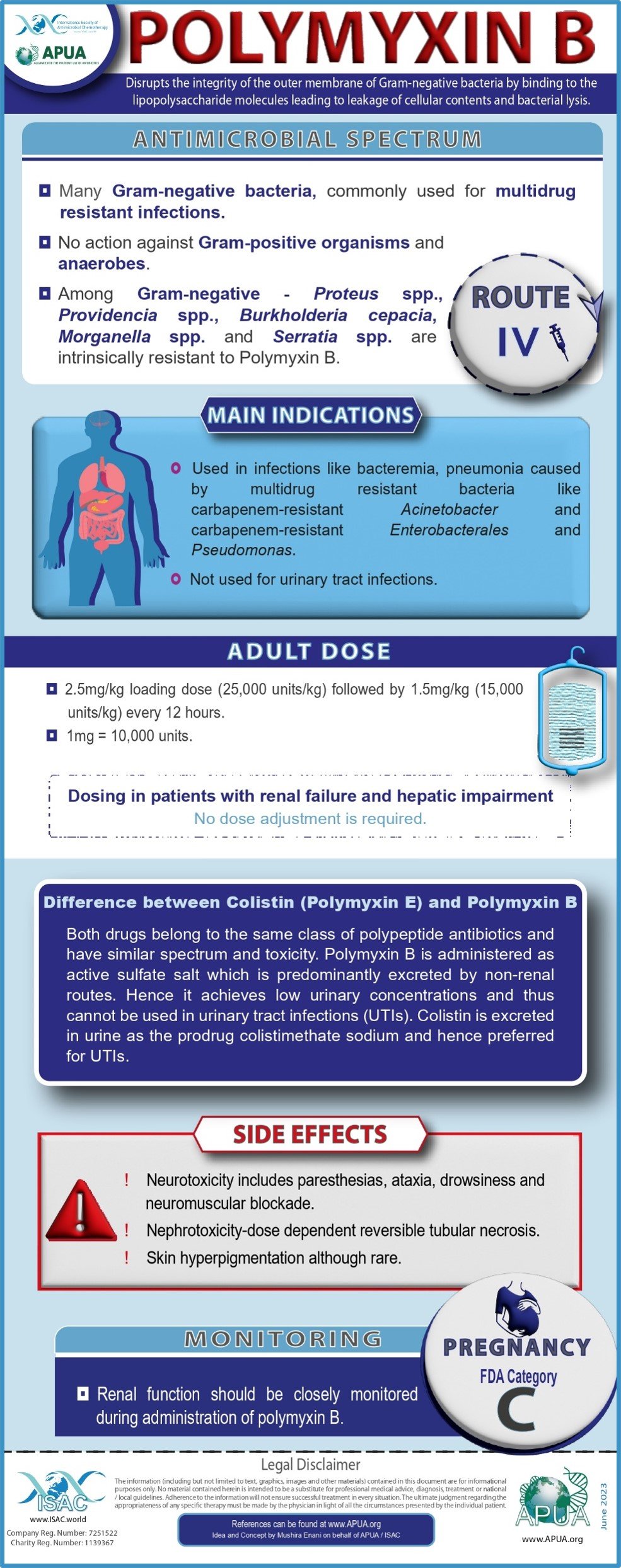
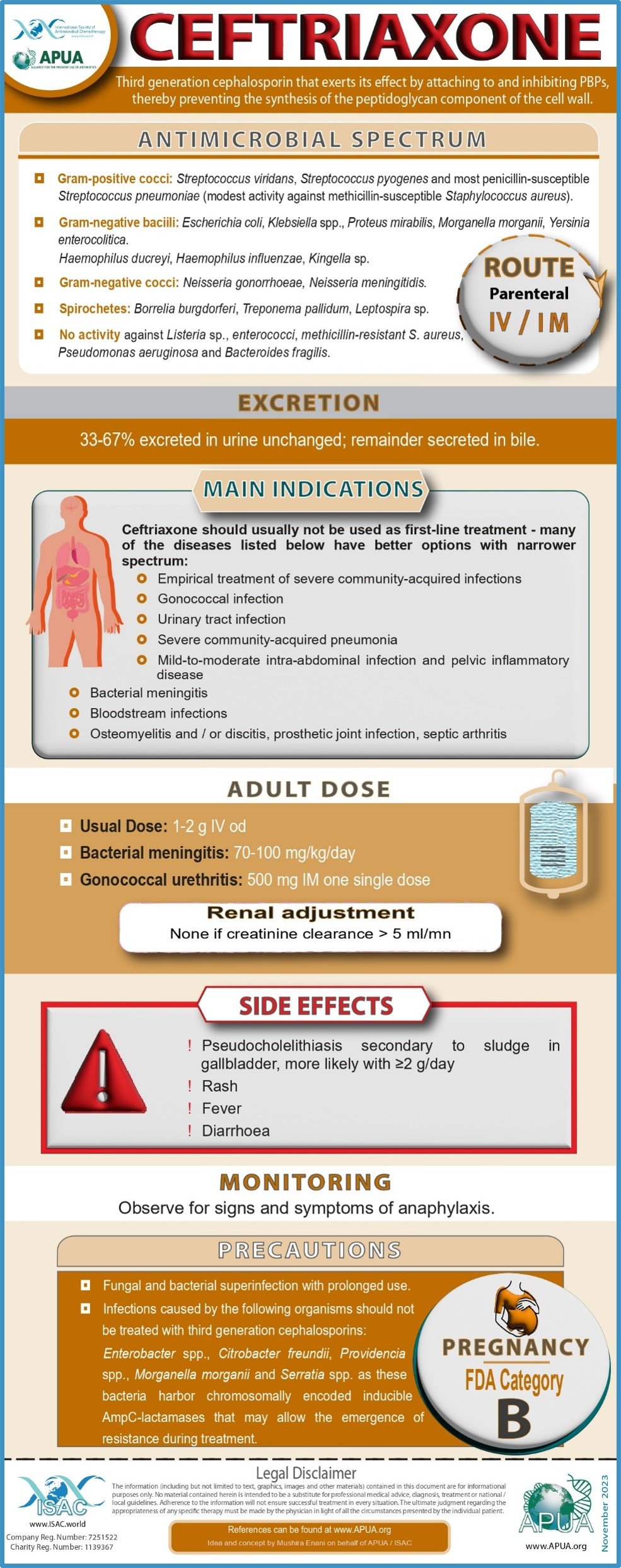
Fosfomycin, Vancomycin, Tigecycline, Ofloxacin, Polymyxin B and Ceftriaxone.
Batch 1
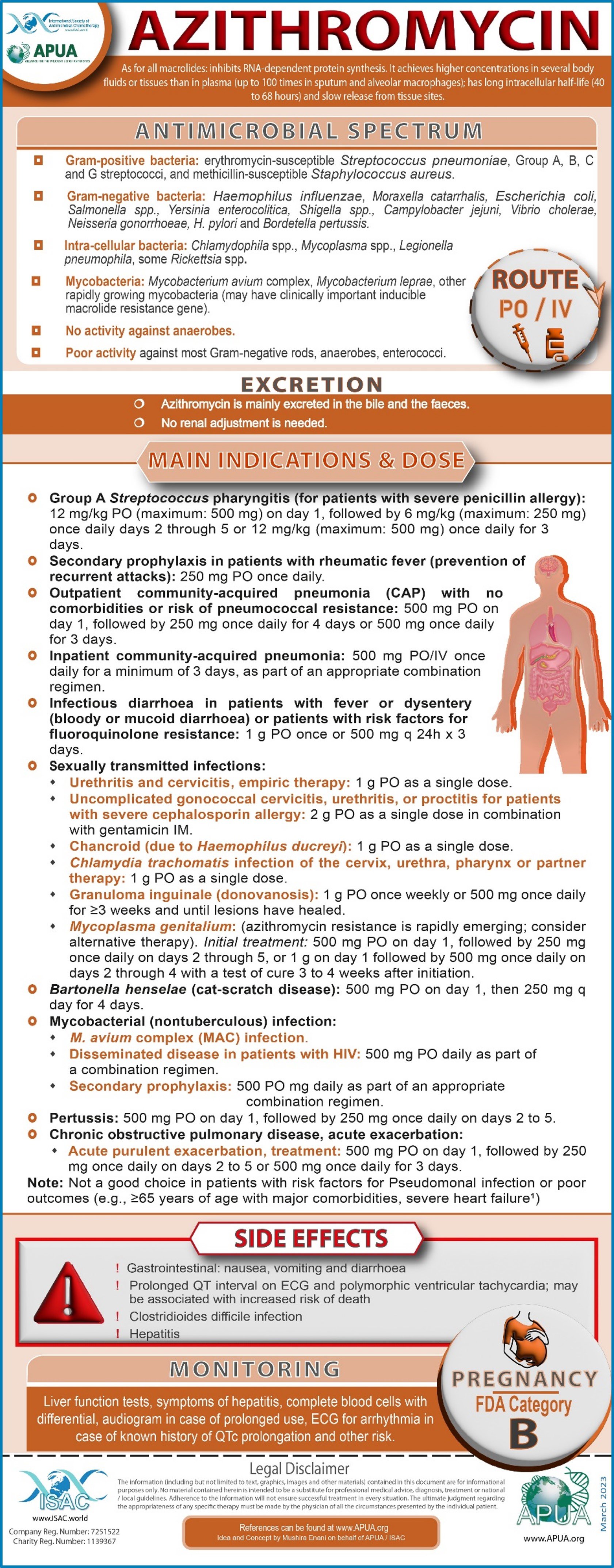
Doxycycline, Azithromycin, Colistin, Meropenem, Imipenem
REFERENCES
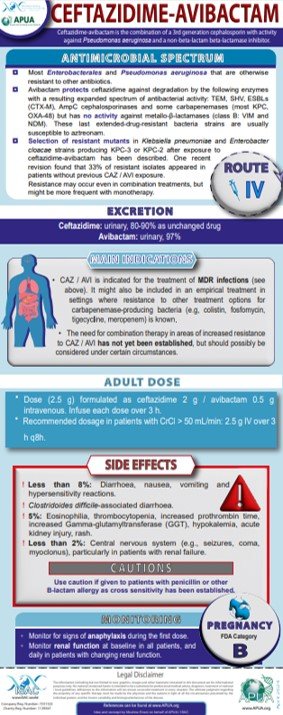
Ceftazidime-Avibactam
Tamma PD et al. Infectious Diseases Society of America 2022 Guidance on the Treatment of Extended-Spectrum β-lactamase Producing Enterobacterales (ESBL-E), Carbapenem-Resistant Enterobacterales (CRE), and Pseudomonas aeruginosa with Difficult-to-Treat Resistance (DTR-P. aeruginosa). Clin Infect Dis. 2022;75:187-212
van Duin D et al. Ceftazidime/Avibactam and Ceftolozane/Tazobactam: Second-generation β-Lactam/β-Lactamase Inhibitor Combinations. Clin Infect Dis. 2016;63:234-41
Matesanz M et al. Ceftazidime-avibactam. Rev Esp Quimioter. 2021;34 Suppl 1:38-40
Di Bella S et al. Resistance to ceftazidime/avibactam in infections and colonisations by KPC-producing Enterobacterales: a systematic review of observational clinical studies. J Glob Antimicrob Resist. 2021;25:268-281
Tamma PD et al. Infectious Diseases Society of America 2023 Guidance on the Treatment of Antimicrobial Resistant Gram-Negative Infections. Clin Infect Dis. 2023:ciad428
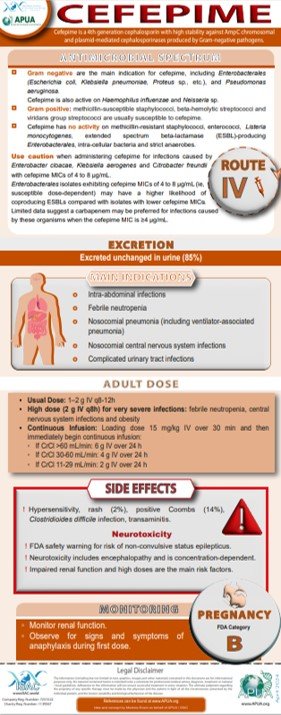
Cefepime
UpToDate: Industry-leading clinical decision supportoDate | Wolters Kluwer
Patel HB et al. The Role of Cefepime in the Treatment of Extended-Spectrum Beta-Lactamase Infections. J Pharm Pract. 2019;32:458-463
Boschung-Pasquier et al. Cefepime neurotoxicity: thresholds and risk factors. A retrospective cohort study. Clin Microbiol Infect. 2020;2:333-339
Tamma PD et al. Infectious Diseases Society of America 2023 Guidance on the Treatment of Antimicrobial Resistant Gram-Negative Infections. Clin Infect Dis. 2023:ciad428
Fosfomycin
Developed by Andrew Nicolson (Dept. of Medical Microbiology and Virology, Aberdeen Royal Infirmary, Aberdeen) on behalf of the APUA Board
Dijkmans AC et al. Fosfomycin: Pharmacological, Clinical and Future Perspectives. Antibiotics (Basel). 2017 Oct 31;6:24
Recommendations to restrict use of fosfomycin antibiotics | European Medicines Agency (europa.eu)). Annnex 2 & Annex 3
Horton JM. 36 - Urinary Tract Agents: Nitrofurantoin, Fosfomycin, and Methenamine. Editor(s): John E. Bennett, Raphael Dolin, Martin J. Blaser, Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases (Eighth Edition), W.B. Saunders 2015, Pages 447-451
Borsa F et al. Comparative pharmacokinetics of tromethamine fosfomycin and calcium fosfomycin in young and elderly adults. Antimicrob Agents Chemother. 1988 Jun;32:938-41
Tigecycline
Developed by Tassabeeh Abdalaziz (Dept. of Medical Microbiology and Virology, Aberdeen Royal Infirmary, Aberdeen) on behalf of the APUA Board
Yaghoubi S et al. Tigecycline antibacterial activity, clinical effectiveness, and mechanisms and epidemiology of resistance: narrative review. Eur J Clin Microbiol Infect Dis. 2022 Jul;41:1003-1022
European Medicines Agency: ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS): the first in the glycylcycline class of antibiotics. Proc (Bayl Univ Med Cent). 2006 Apr;19:155-61
FDA Drug Safety Communication: FDA warns of increased risk of death with IV antibacterial Tygacil (tigecycline) and approves new Boxed Warning. 2017
Ceftriaxone
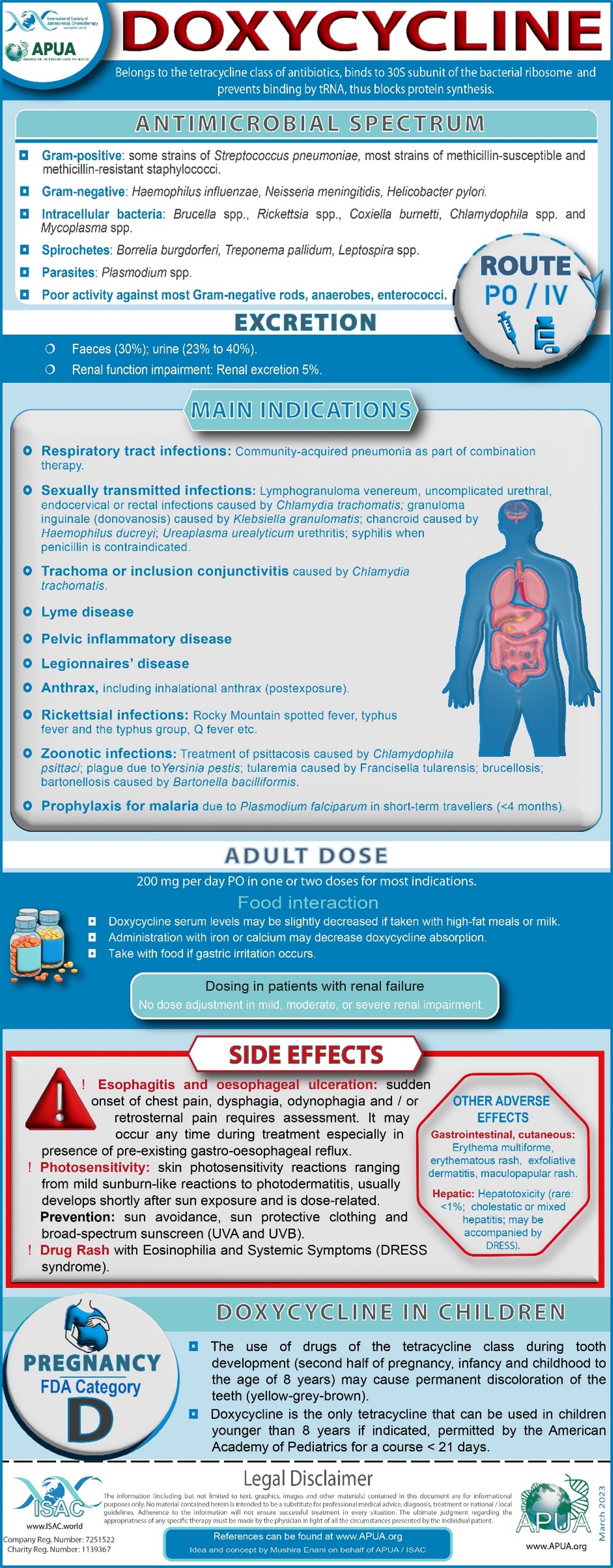
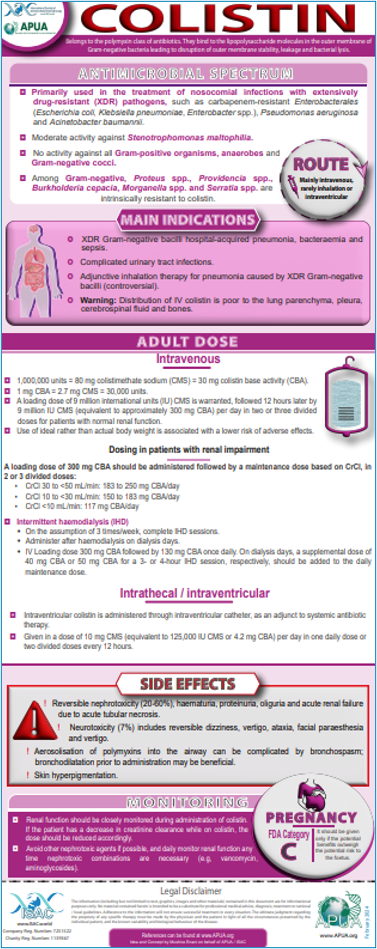
Doxycycline / Azithromycin / Colistin
Hauser, A. R. (2020). Antibiotic Basics for Clinicians (4th ed.). Wolters Kluwer india Pvt Ltd. 2018
Gallagher, J. C., & MacDougall, C. (2016). Antibiotics Simplified (4th ed.). Jones & Bartlett Learning
UpToDate: Industry-leading clinical decision support
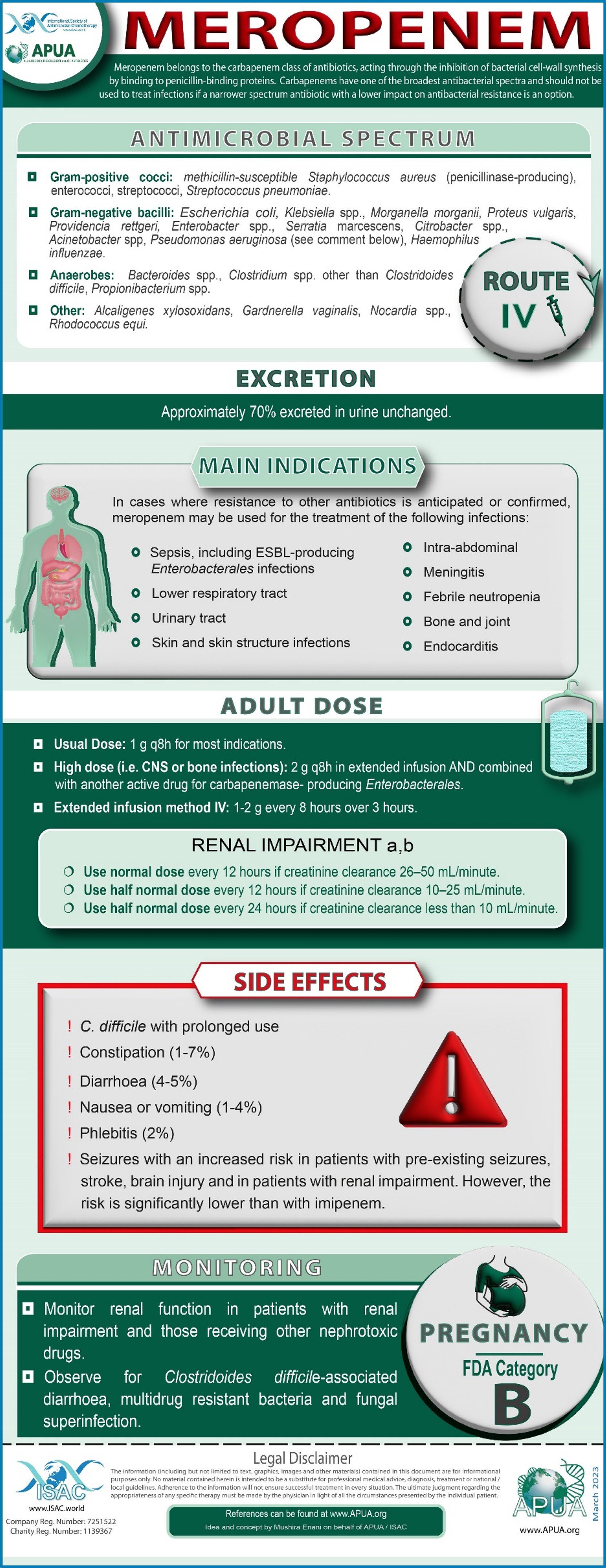
Meropenem
Eljaaly K et al (2018) Impact of carbapenem versus non- carbapenem treatment on the rates of superinfection: A meta-analysis of randomized controlled trials. J Infect Chemother. 2018;24(11):915-920.
Rodríguez-Baño J et al (2018) Treatment of Infections Caused by Extended-Spectrum-Beta-Lactamase-, AmpC-, and Carbapenemase-Producing Enterobacteriaceae. Clin Microbiol Rev. 31(2)
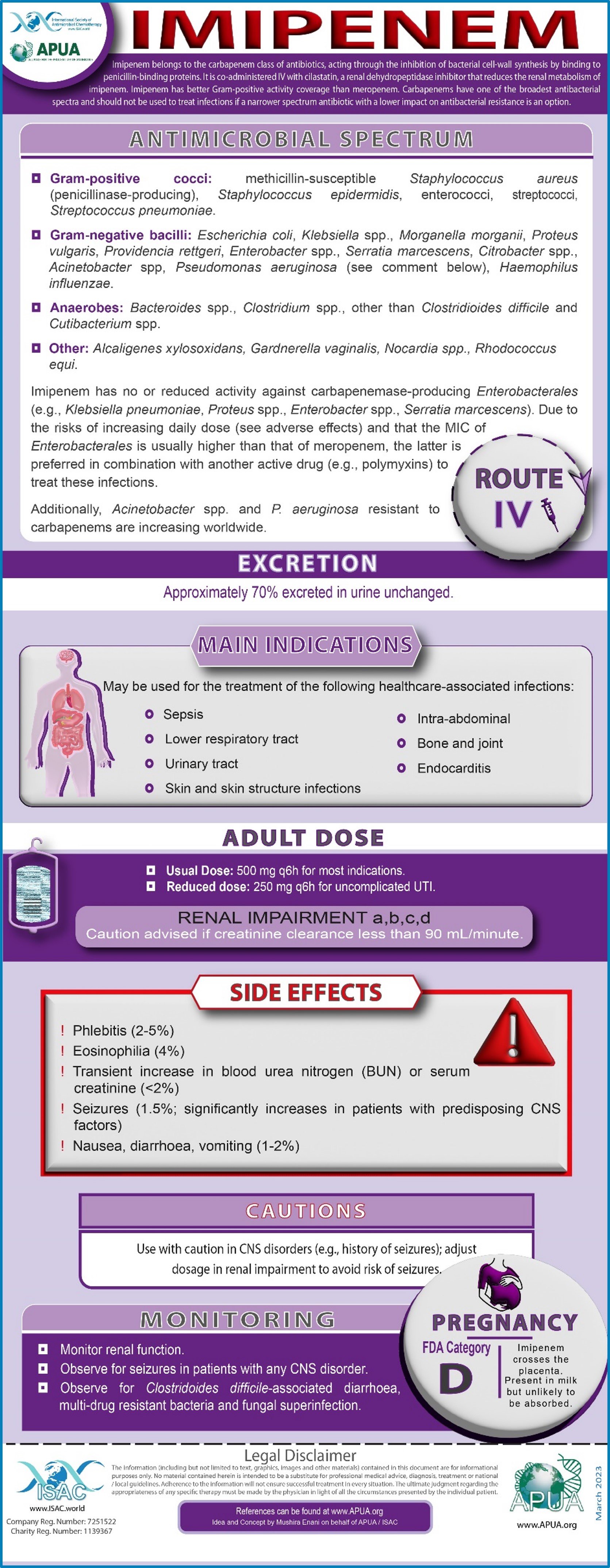
Imipenem